New Advances in Mitral Valve Disease Treatment
In recent years, we’ve seen a surge of innovation for less invasive mitral valve repair and replacement technology. But how do you make sense of the options – and how do you know what’s right for your patient?
When Traditional Surgery Is Not an Option: Transcatheter Mitral Valve Repair
While conventional surgeries are appropriate for some mitral regurgitation (MR) patients, many patients just aren’t healthy enough for surgery. In fact, as many as half of all patients with severe symptomatic MR are not offered surgery due to their age or other comorbidities.
That’s where transcatheter mitral valve repair comes in. The procedure uses a clip device inserted through the femoral vein to repair the mitral valve by grasping both the anterior and posterior mitral valve leaflets, thereby reducing MR.
Transcatheter mitral valve repair is FDA approved for patients with severe primary MR who cannot be offered surgery. What’s more, it offers a low risk of complications and has an average length of stay of only two days.
As the region’s highest volume and most experienced center in transcatheter mitral valve therapies, Sanger Heart & Vascular Institute has been nationally recognized for transcatheter mitral valve repair for more than 12 years. Sanger was one of the first 10 centers in the world to perform it and is the second highest contributor to the pivotal clinical trial EVEREST II.
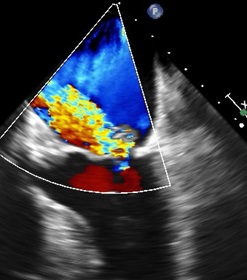
Before: TEE image of posterior leaflet flail
and severe MR
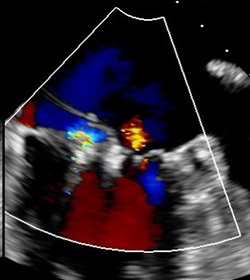
After: TEE image of posterior leaflet flail with
trace MR following procedure
Who’s a Candidate?
Patients best suited for this technology have prolapse or annular dilation with malcoaptation. Despite early experience primarily with simple pathology, transcatheter mitral valve repair has now been applied successfully to a wide variety of pathologies including medial and lateral commissural jets, broad jets, extreme prolapse pathology, MR associated with cardiogenic shock, healed post endocarditis pathology, and failed surgical repair cases.
Patients with secondary MR who fail medical therapy can benefit as well. Often, these patients have limited options. But transcatheter mitral valve repair has been applied successfully to secondary MR as part of the randomized COAPT trial. Sanger was a top 10 contributor to this landmark study, and we continue to enroll patients as part of the continued access registry.
More Transcatheter Treatments
Sanger Heart & Vascular Institute is also one of just a few centers in the Southeast that offers transcatheter treatment to patients with failed mitral bioprosthetic valves and patients with mitral perivalvular leaks following surgical valve replacement.
The Sapien 3 TAVR valve is now approved for mitral valve-in-valve therapy and is placed trans-septally or trans-apically. Similarly, perivalvular leaks can be closed in select patients with catheter delivery of plugs usually through trans-septal access with TEE guidance. Additionally, Sanger is one of the few centers in the Southeast to offer balloon mitral valuloplasty for patients with severe mitral stenosis.
What’s Next for Mitral Valve Disease Treatment?
Exciting new technologies are in development, and many are entering clinical trials.
Sanger is the only center in the Carolinas selected to participate in the CEASE MR study of the Tendyne valve, which is a transcatheter mitral valve replacement technology for secondary MR, beginning in late 2017. This device is implanted trans-apically, and it is uniquely secured through an apical tether, which keeps it stable in position.
First-in-man studies have shown high success, low complications and mortality, and no valvular or perivalvular MR. Transcatheter mitral annular technologies are also in development with early trials being conducted.
Your Referral Center for Mitral Valve Disease Treatment
Sanger Heart & Vascular Institute is the Southeast’s premier referral center for patients with mitral valve disorders and the leading center in minimally invasive surgical repair and transcatheter repair and replacement.
Contact us. Call 704-617-8154 or email SHVIStructuralHeart@AtriumHealth.org.
